Researchers at Chungnam National University, South Korea, have discovered how copper-zinc (CuZn) electrodes adapt and stabilize during electrochemical CO₂ reduction (EC CO₂R). This groundbreaking study led by Professor Youngku Sohn reveals that CuZn electrodes outperform single-metal alternatives, producing valuable hydrocarbons while maintaining efficiency over multiple recycling cycles. These insights could revolutionize carbon capture and conversion technologies, offering a sustainable solution to combat CO₂ emissions and address energy challenges.
A researcher’s team at Chungnam National University has unlocked new potential for copper-zinc (CuZn) electrodes in electrochemical CO₂ reduction (EC CO₂R). This research, led by Professor Youngku Sohn, explores the performance and recyclability of CuZn electrodes, comparing them with single-metal alternatives like copper and zinc, and highlighting their superior catalytic properties. This research was published on October 15, 2024, and was published in Volume 670 of the journal Applied Surface Science.
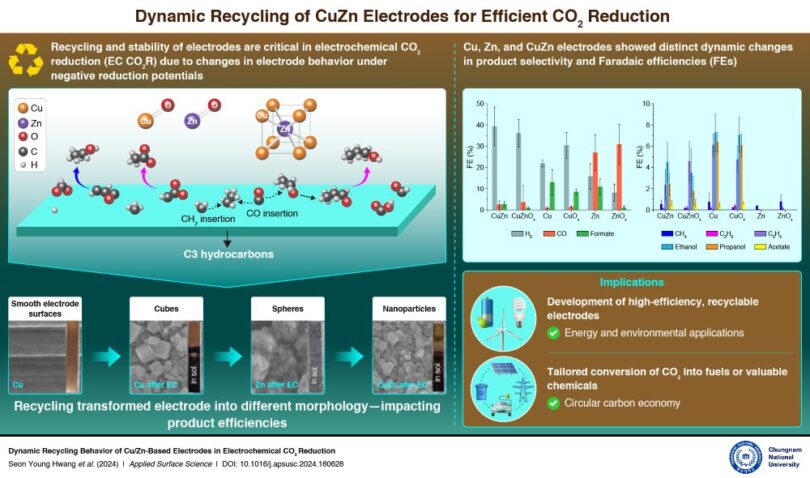
“Electrochemical methods present a promising solution for repurposing CO₂, but electrode stability has always been challenging,” says Prof. Sohn. “Our study shows that CuZn electrodes can stabilize over time through recycling, preserving their catalytic effectiveness and selectivity for valuable hydrocarbons.”
A key highlight of the study was the use of laser techniques to control the oxidation states of the electrodes, allowing researchers to fine-tune their catalytic properties. By analyzing the performance of these electrodes through multiple recycling cycles, the team found that CuZn alloys outperformed single-metal electrodes, providing insights into the importance of surface oxidation states for catalytic efficiency and product selectivity.
The team utilized advanced techniques such as depth-profiling X-ray photoelectron spectroscopy (XPS) to track the changes in oxidation states and compositions of the electrodes. This method revealed that the CuZn electrodes not only stabilize over time but also exhibited a superior ability to maintain selectivity for complex hydrocarbons compared to the single-metal electrodes.
“We also explored the influence of oxidation states on product selectivity,” adds Ms. Seon Young Hwang, a co-author and master’s student in the Department of Chemistry at Chungnam National University. “By controlling the oxidation states, we were able to significantly enhance the electrodes’ performance in reducing CO₂ to valuable products,” She further adds.
This study is particularly relevant for real-world applications, as it enhances the understanding of electrode recyclability and the design of more selective catalysts. The findings could help create high-efficiency CO₂ reduction systems for the conversion of CO₂ into sustainable fuels or valuable chemicals, potentially transforming industries such as energy, manufacturing, and environmental conservation.
“The long-term implications of this work are profound,” says Prof. Sohn. “This research could play a crucial role in developing carbon-neutral industrial processes, contributing to a circular carbon economy by efficiently recycling CO₂ into useful products.”
While the study marks a significant milestone, further research is needed to optimize the scalability of these electrodes for industrial applications. The team’s next step is to examine these electrodes under real-world conditions to understand their capabilities better.
This groundbreaking work provides a pathway to more stable and efficient catalytic systems, advancing the global goal of achieving sustainable carbon management solutions.